Enzyme, Protein Biology, COVID-19 
Proteinase K, Recombinant, PCR Grade
- Catalog Number : ER1021
- Number : ER1021
-
Size:
Qty : - Price : Request Inquiry
Introduction
General Information
| Application | Cell |
|---|---|
| Assay principle | Reconstitute lyophilized recombinant proteinase K in 50% glycerol solution and store at -20°C.Please avoid repeated freeze-thaw cycles after reconstitution. |
| Purity | >95% (SDS-PAGE) |
| Storage instruction | The lyophilized recombinant proteinase K has been sterilized and filtered through a 0.22 µm filter, which can maintain its activity for a long time at 2-8°C. It is recommended to store at -20°C for long-term storage. |
Application
Viral DNA/RNA isolation
DNA/RNA Experiment, for DNA/RNA Isolation
Information
Source: Yeast
Physical Appearance: White or light beige lyophilized powder
Bioactivity: ≥30U/mg
E.C. No.: 3.4.21.64
Quality control: DNase and RNase Non-detection
Expiry date: 24 months.
Activators
1-5 mM Ca2+ is required for activation. When calcium is removed from the enzyme (addition of EDTA) 25% of the catalytic activity is lost. However, if the EDTA-Ca2+ complex is removed from the enzyme solution by gel filtration, a total of 80% of the enzyme activity is lost and only a small activation will occur upon addition of excess Ca2+ to the Ca2+-free enzyme.
Inhibitors
Proteinase K is inhibited by DIFP or PMSF (the latter used at final concentration 5 mM). It is partly inactivated, but not inhibited, by EDTA (see Activators). Proteinase K is not inhibited by iodoacetic acid, the trypsin-specific inhibitor TLCK, the chymotrypsin specific inhibitor TPCK and phloromercuribenzoate.

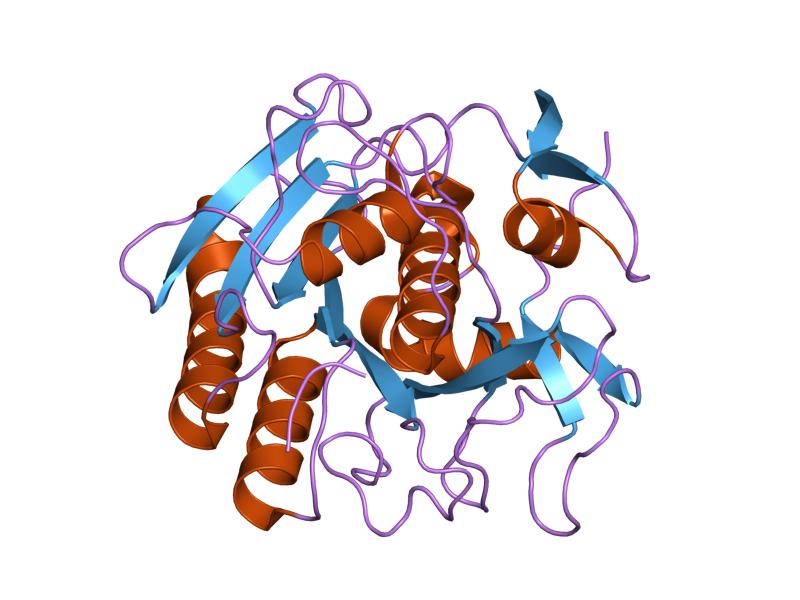





.png)