Neutralizing Antibody Detection
Virus neutralization remains the gold standard for determining antibody efficacy. Therefore, a high-throughput assay to measure SARS-CoV-2 neutralizing antibodies is urgently needed for COVID-19 serodiagnosis, convalescent plasma therapy, and vaccine development.
Pseudoviral Neutralizing Antibody Detection Platform
Principle of Pseudovirus Neutralizing Antibody Assay
In the Pseudovirus Neutralizing Antibody Assay, the inhibition of viral entry into cells by Neutralize Antibody is correlated to the decreased levels of reporter gene (usually use luciferase) signals in the cells. This method is superior to the conventional assay because of its simplicity, higher sensitivity and accuracy, suitability for high-throughput experiments. In addition, pseudovirus is used during the test. Therefore, this method could be used as an alternative for safely conducting serologic studies in a rapid response in assessing the threat posed by SARS-CoV-2.
.png)
Strategy of Pseudovirus Neutralizing Antibody Assay
To Set up a Pseudovirus Neutralizing Antibody Assay, the necessary requirements are 1) Pseudovirus, 2) Target Cell Line, 3) Standard Neutralizing Antibody, and 4) Enhancers.
(1)
Pseudovirus
Non-replication for safety, Spike protein for infection and Reporter genes for analysis
Non-replication Lentiviral Packaging System for Safety
ACE Biolabs suggest to us the lentiviral packaging system to generate SARS-CoV-2 pseudovirus.
Lentivirus is regarded as a biosafety level 2 material and safe to use due to its modified features (deletion of a number of accessory virulence genes , minimal genome of the viral particles, non-replicating and self-inactivation features), making it incapable of producing virus once infected into the host cell.
Pseudovirus package use the SARS-CoV-2 (2019-nCoV, COVID-19 virus) S protein as a surface capsid glycoprotein and containing GFP and/or Luciferase reporter genes as a pseudovirus model. The virus can infect cell lines overexpressing the ACE2 gene. Researchers can determine the infection efficiency by observing GFP through a microscope and detecting the value of Luciferase.
.png)
SARS-CoV-2 Spike Protein as a Surface Capsid Glycoprotein for Infection
First sequenced Spike Protein (NC_045512.2) is most frequently used for Pseudovirus Neutralizing Antibody Assay. And "Bald" lentivirus that does not contain any envelope protein can be used as a negative control. However, recently reports show that mutation of S protein improves infectivity or/and decreases sensitivity to neutralizing antibodies (ref: https://doi.org/10.1038/s41392-020-00302-8).
.png)
Wang, L., Wang, L. & Zhuang, H. Profiling and characterization of SARS-CoV-2 mutants' infectivity and antigenicity.
Sig Transduct Target Ther 5, 185 (2020). https://doi.org/10.1038/s41392-020-00302-8
D614G mutation improved infectivity -
.png)
.png)
A spike protein mutation D614G became dominant in SARS-CoV-2 during the COVID-19 pandemic. Plante, J.A. et al. reported that D614G enhances replication on human lung epithelial cells and primary human airway tissues through an improved infectivity of virions. ref: https://doi.org/10.1038/s41586-020-2895-3
C-ter modification improved infectivity -
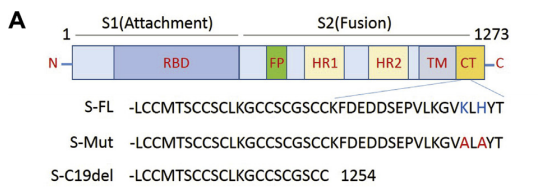
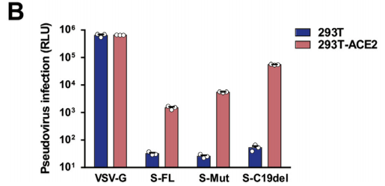
Hu J. et al. generated pS-Mut plasmid bearing two mutations that destroy the endoplasmic reticulum (ER) retrieval signal (“KxHxx” motif) in the CT domain of the S protein and pS-C19del plasmid lacking the C-terminal 19 amino acids. The highest viral titer was observed in S-C19del pseudotyped virus, suggesting that the this mutant S facilitates lentivirus packaging. ref: https://dx.doi.org/10.1016%2Fj.gendis.2020.07.006
Reporter genes for analysis
Commonly used reporter genes that induce visually identifiable characteristics usually involve fluorescent and luminescent proteins. Examples include the gene that encodes green fluorescent protein (GFP), red fluorescent protein (RFP) and the enzyme luciferase.
(2)
Cell line: express ACE2 receptor
An important aspect of Pseudovirus Neutralizing Antibody Assay lies in the basic mechanism of viruses invading cells. Cell line for Pseudovirus Neutralizing Antibody Assay can be natural cells expressing viral S protein receptors, or use artificially modified special cell types that can stably express S protein receptor genes. The advantage of using artificially modified cell lines is that certain cell surface receptors can be expressed at a high level according to the research needs, especially those receptor types that mediate virus invasion into cells.
Natural cells expressing viral S protein receptors
Ma D. et al. determined the expressions of SARS-CoV-2 receptor angiotensin-converting enzyme 2 (ACE2) and type II transmembrane serine protease (TMPRSS2) genes in human cells. https://doi.org/10.1038/s41433-020-0939-4
.png)
Stable cell line express ACE2 receptor genes
ACE2-3xFLAG-HEK293T stable cell line : The ACE2-3xFLAG-HEK293T stable cell line was obtained from lentivirus infected and multiple rounds of Puromycin screening. The lentivirus was packaged with pLV-ACE2-3FLAG plasmid, concentrated and purified, and then infected with HEK293T cells.
Common cell line used for express ACE2 including HEK293T, Hela, BHK-21 and etc. ACE Biolabs packaged lentivirus with pLV-ACE2-3FLAG plasmid. This ACE2-lentivirus is available to infect various cell lines. Stable cell lines that express ACE2 receptor are further generated with selection by puromycin.
(3)
Standard Neutralizing Antibody
|
Anti-SARS-CoV-2 S-hIgG1 Neutralizing Antibody
Anti-SARS-CoV-2 S-hIgG1 Neutralizing Antibody can block Human ACE-2 and S-trimer Protein interaction. |
Anti-SARS-CoV-2 S-hIgM Neutralizing Antibody
Anti-SARS-CoV-2 S-hIgM Neutralizing Antibody can block Human ACE-2 and S-trimer Protein interaction. |
Anti-SARS-CoV-2 S-hIgA Neutralizing Antibody
Anti-SARS-CoV-2 S-hIgA Neutralizing Antibody can block Human ACE-2 and S-trimer Protein interaction. |
.jpg)
Non-viral Neutralizing Antibody Detection Platform
PRINCIPLE OF THE ASSAY
This assay employs the competitive inhibition enzyme immunoassay technique. The microtiter plate provided in this kit has been pre-coated with RBD. Standards or samples are added to the appropriate microtiter plate wells with Horseradish Peroxidase (HRP) conjugated ACE2. The competitive inhibition reaction is launched between with HRP-ACE2 and SARS-CoV-2 neutralizing antibody in samples. A substrate solution is added to the wells and the color develops in opposite to the amount of SARS-CoV-2 neutralizing antibody in the sample. The color development is stopped and the intensity of the color is measured. %20Neutralizing%20Antibody%20ELISA%20Kit-01.png)
DETECTION RANGE
9.75 ng/ml-10000 ng/ml.
SENSITIVITY
The minimum detectable dose of human SARS-CoV-2 neutralizing antibody is typically less than 9.75 ng/ml. The sensitivity of this assay, or Lower Limit of Detection (LLD) was defined as the lowest human SARS-CoV-2 neutralizing antibody concentration that could be differentiated from zero.

.png)